Feature Story
Health Center Today, June 29, 2010
Four UConn Stem Cell Lines Approved for Use in Federally Funded Research
By David Bauman
Ren-He Xu, director of the Stem Cell Core Laboratory at the UConn Health Center.
Photo by Al Ferreira
Four human embryonic stem cell lines derived by University of Connecticut scientists have been approved for use in federally funded research and added to the National Stem Cell Registry by the National Institutes of Health.
The four UConn stem cell lines – identified as CT1, CT2, CT3, and CT4 – were derived in the UConn Health Center’s Stem Cell Core Laboratory using public funds provided by the State of Connecticut and its stem cell initiative. They are the first stem cell lines from a Connecticut institution to receive federal approval.
“To have the only four human embryonic stem cell lines from Connecticut in the NIH registry is a major achievement,” says Marc Lalande, senior associate dean for research planning and coordination at the UConn Health Center, director of UConn’s Stem Cell Institute, and professor and chairman of the medical school’s genetics and developmental biology department. “It justifies that we’ve used the state’s investment and really moved to the national stage in terms of human embryonic stem cell research.”
The embryos used for the creation of all four CT lines were originally generated for reproductive purposes and subsequently donated for research purposes following consent of the donors, says Ren-He Xu, director of UConn’s human embryonic stem cell core laboratory. Some of the lines have already been distributed to stem cell researchers at UConn and beyond, and used in several publications of research discoveries supported by the state before the NIH registry applications were filed, he adds.
“I am very happy that the UConn-created stem cell lines have now become eligible for use by any researcher supported by U.S. federal funding agencies such as NIH and NSF,” says Xu, a developmental biologist and expert in growing human embryonic stem cells lines. “It is wonderful to think that future discoveries by federally-funded scientists might be based on the lines developed here at UConn.”
Embryonic stem cells can grow into every type of body tissue and thus are considered a research tool that could lead to cures for many illnesses. But not all stem cell lines serve equally well for this. There is a great deal of variation in quality, based on their genetic makeup and conditions under which they are handled.
Approval of new stem cell lines by the NIH, the government’s prime medical research agency, gives scientists greater access to a variety of stem cell lines – each from one donated embryo – to study embryonic development, explore new treatments for diseases, and test drugs.
Previously they could do such work only under rules the Bush administration had imposed that limited funding to 21 stem cell lines (so-called Bush lines) created before August 2001, or by using private or state funds to study the non-Bush lines. For example, UConn’s Xu announced the creation of lines CT1 and CT2 last January, joining an elite group of universities with labs creating stem cell lines. But those lines were not eligible for use by federally-funded researchers before the NIH registration.
Last year President Obama lifted the restrictions on federal funding for human embryonic stem cell research and called on the NIH to develop new guidelines to open federal funding to newly established lines. The agency issued detailed guidelines last summer, including requirements that couples who donate embryos provide written consent for their use for research purposes, and that they not receive payment or expect medical or financial benefits later.
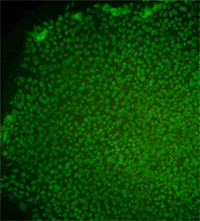
Immunostained image for the CT3 human embryonic stem cell line.
Image supplied by Ren-He Xu
Xu says it took “considerable” time to assemble the necessary paperwork before submitting the applications for the CT stem cell lines to the NIH for approval in September 2009. He recalls that a NIH committee of scientists and ethicists “took many months to ask more questions and request more documents. “At least five times,” he says.“You have to be very careful and very professional,” he adds. “They are very picky.”
NIH approval of the four CT lines brings to 75 the number of lines that have been approved so far under the new guidelines. An additional 83 lines are awaiting review. Approval of new lines will provide scientists more options to address their research needs. Additional lines will expand the genetic diversity, and those derived from embryos with genetic diseases will be fitting tools to study the corresponding diseases.
Srdjan Antic, assistant professor of neuroscience at the Health Center, began using stem cell lines from the UConn stem cell core lab a year ago after being awarded a state “seed grant” by the Connecticut Stem Cell Research Advisory Committee to jump-start his stem cell research.
“I am happy to report that I have had eight rounds of stem cell differentiation, followed by immunohistochemical and electrophysiological characterization of healthy, good-looking post-mitotic neurons,” Antic wrote in a letter to Xu. Antic used CT2 lines in two rounds of differentiation, while the rest of his work was done with another NIH-approved line.
Antic was awarded a second state grant last month, to establish a neuronal physiology and chemistry core facility to assess the functionality of neurons generated from stem cells in sample cultures provided by scientists from around the state. “The success of the second grant relied upon showing preliminary data of good quality,” Antic’s letter continued. “All this would not have been possible without the excellent CT2 lines, in expansion cultures, ready to use, for the start-up of our differentiation experiments.”


