Feature Story
Health Center Today, October 19, 2010
UConn Scientists Generate Functional Nerve Cells from Adult Skin Cells
By David Bauman
The Health Center's Dr. Ren-He Xu is co-leader of a team that has generated functional nerve cells from adult skin cells.
Photo by Al Ferreira
Scientists at the UConn Health Center have successfully converted stem cells derived from the adult skin cells of four humans into region-specific forebrain, midbrain, and spinal cord neurons (nerve cells) with functions. The research is a key step toward realizing the cells’ potential to treat various neurodegenerative diseases.
The UConn team, led by Dr. Ren-He Xu, director of the Health Center’s Stem Cell Core facility, and Dr. Xuejun Li, a neural scientist in the Neuroscience Department, recently published a paper describing how they used cell reprogramming protocols to first transform the adult tissue into “induced pluripotent stem cells” that are all but identical to embryonic stem cells.
This involved treating the adult skin cells with a specialized culture that caused them to regress in their development to an embryonic-like “pluripotent” state, capable of differentiating into any of the many tissue types in the body. The researchers then exposed these reprogrammed human cells (hiPSC) to a series of chemical mixtures to drive them into becoming specialized neuronal cells.
As part of the same research project, Xu’s team also directed two previously established human embryonic stem cell (hESC) lines into neuronal cells, in order to determine whether there were meaningful differences between using human embryonic stem cells and human induced pluripotent cells.
UConn Health Center scientists who collaborated with Xu and Li on the research included Lixia Yue (a physiologist) and Alexander Lichtler (a geneticist) from the departments of Cell Biology and Regenerative Sciences respectively. Their study was published in PLoS ONE, an international, peer-reviewed online journal of the non-profit Public Library of Science (PLoS).
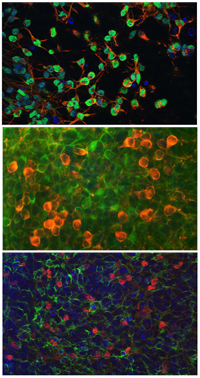
Functional neurons formed by human induced pluripotentstem (iPS) cell line TZ1. Top: glutamatergic neurons; middle: dopaminergic neurons; and bottom: motor neurons.
Images provided by Dr. Ren-He Xu
The rapid development of iPSCs since they were first produced in 2006 has generated tremendous interest among researchers. The ability to take easily obtainable skin cells and potentially make any tissues in the body eliminates the need to destroy human embryos to obtain hESC. Also, because human iPS cells have the same genetic background as the person they come from, they enable scientists to create perfectly matched cells for patient-specific therapies that would be immune to rejection.
Yet the research community remains uncertain whether iPS cells have the same quality as hESC. Some biologists suspect that the tissue of origin influences the iPS cells’ ability to develop into different cell lineages; others are not so sure. What they do agree on is that better understanding the mechanisms underlying such epigenetic memory and its consequences is vital to using iPS cells in a clinical setting.
In their PLoS ONE paper, the UConn researchers contend: "Our results demonstrate that hiPSC, regardless of how they were derived, can differentiate into a spectrum of neurons with functionality, which supports the considerable value of hiPSC for study and treatment of patient-specific neural disorders."
Although the differentiation of embryonic stem cells into neuronal cells has been well established in other labs, Li notes that the UConn research is the first to focus on the ability of human iPSC to create functional neurons in region-specific areas of both the brain and spinal cord.
A key step for using stem cells as therapy in neurological diseases, says Xu, must be developing the ability to direct their differentiation into neural lineages and then to specific neuronal types that are affected by different diseases such as Parkinson’s or Alzheimer’s disease.
While the UConn study showed that the "efficiency of neural differentiation" among the hiPSC lines was mixed, Xu notes that the process resembled that of the hESC lines in morphology, gene expression, and the chemical signals and conditions needed to regulate how the stem cells programmed to differentiate into neural cells.
"Together," he said, "our work has demonstrated that hiPSC, regardless of how they are derived, can generate a spectrum of region-specific neurons."


